
Founded in 2006 by R&D leaders from industry
Biometric sensor technology engineered in the United States by a world-class team of experts
Pioneers in the field of biometric sensors with 150+ awarded patents
Foundational for accurate biometric monitoring during exercise and everyday life activities
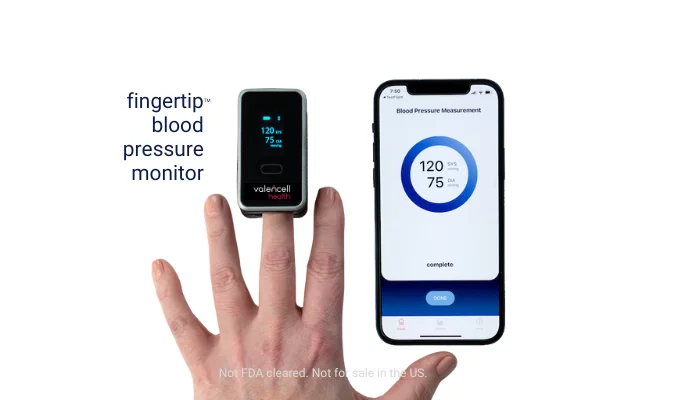
First OTC, cuffless, calibration-free, clinically proven accurate Fingertip™ Blood Pressure Monitor
Leading the way in digital health solutions for hypertension / chronic disease management
Award-winning biometric sensing inventions found in over 90 leading brands
Proliferated in tens of millions of wearables worldwide - Samsung, Suunto, Bose, Jabra, Sony ++
VALENCELL, INC.
Raleigh, North Carolina (NC), United States
Healthcare
Products & Services
People
News
About
Products & Services
Valencell Health Fingertip™ Blood Pressure Monitor -- The future of hypertension management is at your FINGERTIP.
Valencell, Inc.
Blood Pressure, Uncuffed™
Valencell Health’s Fingertip™ Blood Pressure Monitor is the first OTC, cuffless, calibration-free BP spot-check solution at the finger. Easy-to-use, clinically proven accurate, one size and with one button operation, the Fingertip™ BPM is compact, portable, comfortable, and convenient to use at home or on-the-go. Confidently manage your health with the connected app that records and tracks BP history, sets reminders to take BP regularly and downloads and shares data with your healthcare provider. Engineered in the United States using photoplethysmography (PPG) sensor technology and proprietary algorithms trained on 20,000+ unique datasets collected from over 10,000 patients worldwide over many years.
Blood pressure measurement will change more in the next 5 years than it has in the last 100.
Not FDA cleared. Not for sale in the US.
Valencell Health’s Fingertip™ Blood Pressure Monitor is the first OTC, cuffless, calibration-free BP spot-check solution at the finger. Easy-to-use, clinically proven accurate, one size and with one button operation, the Fingertip™ BPM is compact, portable, comfortable, and convenient to use at home or on-the-go. Confidently manage your health with the connected app that records and tracks BP history, sets reminders to take BP regularly and downloads and shares data with your healthcare provider. Engineered in the United States using photoplethysmography (PPG) sensor technology and proprietary algorithms trained on 20,000+ unique datasets collected from over 10,000 patients worldwide over many years.
Blood pressure measurement will change more in the next 5 years than it has in the last 100.
Not FDA cleared. Not for sale in the US.
Valencell, Inc. Biometrics Lab
Valencell, Inc.
Located in Raleigh, NC, the Valencell Biometrics Laboratory is one of the most efficient human research facilities in the world, contributing to the analysis and development of many health studies and technological advances. Our capabilities range from pre-clinical consultation, all the way to full assessment and validation of devices, leveraging our expert team of Certified Healthcare Clinical Research Specialists, ACSM Certified Exercise Physiologists, Certified Personal Trainers, and NSCA Certified Strength and Conditioning Specialists.
Research Studies & Data Analytics
Our in-lab or field-based validation testing provides access to data and assessment of physiological biometrics as applied to exercise, activities of daily living, biotech, and medical situations. We specialize in conducting “pre-clinical” testing to address any study design challenges or device usage issues prior to a clinical trial, and can implement a wide variety of protocols. Whether you already have a research study designed and need the lab space and staff to carry out the testing, or simply need an unbiased external testing site, we understand the importance of speed and accuracy during this phase and maintain a diverse subject pool for all research recruitment needs.
Wearable Technology Assessment & Validation
Benefit from our well-renowned facility for validating both consumer and medical-grade wearable devices. Our lab assures data and metrics are valid and reliable and can help coordinate both internal and external trials. Metrics typically assessed include but are not limited to: heart rate, heart rate variability, blood pressure, temperature, respiration, blood glucose, blood lactate, step rate and step count.
Consulting & Guidance
Partner with us for guidance on metrics for any application including mobile health and fitness, validation trials, protocol design and physiological assessment in clinical settings.
SERVICES OFFERED
Wearable Technology Development and Validation
VBL specializes in wearable technology development and assessment of device accuracy and reliability.
We handle both consumer and medical-grade wearable devices. Metrics typically assessed include but are
not limited to: heart rate; heart rate variability; blood pressure; caloric expenditure; oxygen utilization;
substrate (carbohydrate/fat) utilization; temperature; respiration; blood glucose; blood lactate; oxygen
saturation; and altitude simulation.
Internal research to guide research and development efforts may include:
• Assure data and metrics are valid and reliable
• Guide research and development with ongoing data collection
• Recruitment and organization of beta testers for feedback of product during the research and development process
• Design of methods to assess metrics during the R&D process
• Statistical analysis and evaluation
• Organization of data and results for presentation, marketing, and investor support
• Internal trials can be performed at your facility, at our laboratory and/or in the field
Once internal trials for validation and reliability are complete, external trials can be initiated. VBL can
coordinate external research efforts by:
• Arranging for trial location or work with sites already in place
• Providing representation during data collection to assure proper methodology
• Developing site contract and point of contact during development, data collection and publication
for conference and journal submission
• Analyzing and presenting results
Research Studies
Beyond wearable device testing, VBL is fully capable of a variety of human research study implementations.
Perhaps you already have a research study designed but need the lab space and staff to carry out the
testing, or simply need an unbiased external testing site – our lab may be able to help. Our team has
backgrounds and experience in both clinical and academic research study design and implementation.
The lab is accustomed to implementing a wide variety of protocols from high intensity exercise to activities
of daily living, both in-lab and field-based. We have worked with all types of participants and are
accustomed to modifying procedures as needed for a given population. The ability to customize testing
protocols to match the intended user experience is a key characteristic of VBL.
Consulting
Whether you need the testing and implementation conducted or simply need some advice, we are here to
help. The VBL offers consulting for research and development of health and fitness metrics for
implementation into industry. The laboratory can provide guidance on metrics for any application including
mobile health and fitness, validation trials, protocol design and physiological assessment in clinical
settings. Guidance can take the form of simple presentation to complete development of internal and
external trials for research and development, validation and implementation.
EQUIPMENT AVAILABLE
VBL has the following equipment available for use. Additional equipment can be procured as needed. The
lab also has access to a nearby pool for any in-water testing needs. The lab staff are also CPR/AED
certified through the American Red Cross and we have an AED and first aid kit on site in the lab.
Reference Testing Equipment:
• Polar H10 Chest Straps
• Automatic and Manual BP Cuffs
• Cadence Sensor
• SpO2 Monitor
• Blood Glucose Monitor
• Blood Lactate Monitor
• Thermometer
• Core Temperature Monitor
• Altitude Simulator
• Modus StepWatch4
• Spotlight (mimic sunlight indoors)
Gym Equipment:
• Epic Treadmill (2)
• Livestrong Stationary Bike
• Dumbbells
• Kettlebells
• Medicine Balls
• Slam Balls
• Yoga/Exercise Ball
• Rowing Machine
• Bench
• Mats
• Aerobic Step Bench
• Jump Ropes
Research Studies & Data Analytics
Our in-lab or field-based validation testing provides access to data and assessment of physiological biometrics as applied to exercise, activities of daily living, biotech, and medical situations. We specialize in conducting “pre-clinical” testing to address any study design challenges or device usage issues prior to a clinical trial, and can implement a wide variety of protocols. Whether you already have a research study designed and need the lab space and staff to carry out the testing, or simply need an unbiased external testing site, we understand the importance of speed and accuracy during this phase and maintain a diverse subject pool for all research recruitment needs.
Wearable Technology Assessment & Validation
Benefit from our well-renowned facility for validating both consumer and medical-grade wearable devices. Our lab assures data and metrics are valid and reliable and can help coordinate both internal and external trials. Metrics typically assessed include but are not limited to: heart rate, heart rate variability, blood pressure, temperature, respiration, blood glucose, blood lactate, step rate and step count.
Consulting & Guidance
Partner with us for guidance on metrics for any application including mobile health and fitness, validation trials, protocol design and physiological assessment in clinical settings.
SERVICES OFFERED
Wearable Technology Development and Validation
VBL specializes in wearable technology development and assessment of device accuracy and reliability.
We handle both consumer and medical-grade wearable devices. Metrics typically assessed include but are
not limited to: heart rate; heart rate variability; blood pressure; caloric expenditure; oxygen utilization;
substrate (carbohydrate/fat) utilization; temperature; respiration; blood glucose; blood lactate; oxygen
saturation; and altitude simulation.
Internal research to guide research and development efforts may include:
• Assure data and metrics are valid and reliable
• Guide research and development with ongoing data collection
• Recruitment and organization of beta testers for feedback of product during the research and development process
• Design of methods to assess metrics during the R&D process
• Statistical analysis and evaluation
• Organization of data and results for presentation, marketing, and investor support
• Internal trials can be performed at your facility, at our laboratory and/or in the field
Once internal trials for validation and reliability are complete, external trials can be initiated. VBL can
coordinate external research efforts by:
• Arranging for trial location or work with sites already in place
• Providing representation during data collection to assure proper methodology
• Developing site contract and point of contact during development, data collection and publication
for conference and journal submission
• Analyzing and presenting results
Research Studies
Beyond wearable device testing, VBL is fully capable of a variety of human research study implementations.
Perhaps you already have a research study designed but need the lab space and staff to carry out the
testing, or simply need an unbiased external testing site – our lab may be able to help. Our team has
backgrounds and experience in both clinical and academic research study design and implementation.
The lab is accustomed to implementing a wide variety of protocols from high intensity exercise to activities
of daily living, both in-lab and field-based. We have worked with all types of participants and are
accustomed to modifying procedures as needed for a given population. The ability to customize testing
protocols to match the intended user experience is a key characteristic of VBL.
Consulting
Whether you need the testing and implementation conducted or simply need some advice, we are here to
help. The VBL offers consulting for research and development of health and fitness metrics for
implementation into industry. The laboratory can provide guidance on metrics for any application including
mobile health and fitness, validation trials, protocol design and physiological assessment in clinical
settings. Guidance can take the form of simple presentation to complete development of internal and
external trials for research and development, validation and implementation.
EQUIPMENT AVAILABLE
VBL has the following equipment available for use. Additional equipment can be procured as needed. The
lab also has access to a nearby pool for any in-water testing needs. The lab staff are also CPR/AED
certified through the American Red Cross and we have an AED and first aid kit on site in the lab.
Reference Testing Equipment:
• Polar H10 Chest Straps
• Automatic and Manual BP Cuffs
• Cadence Sensor
• SpO2 Monitor
• Blood Glucose Monitor
• Blood Lactate Monitor
• Thermometer
• Core Temperature Monitor
• Altitude Simulator
• Modus StepWatch4
• Spotlight (mimic sunlight indoors)
Gym Equipment:
• Epic Treadmill (2)
• Livestrong Stationary Bike
• Dumbbells
• Kettlebells
• Medicine Balls
• Slam Balls
• Yoga/Exercise Ball
• Rowing Machine
• Bench
• Mats
• Aerobic Step Bench
• Jump Ropes
People
News
Valencell Health Fingertip™ Blood Pressure Monitor - Video in Arabic
Valencell Health Video (Arabic) https://youtu.be/HJYbgYrTGVk
23 Jan 2024
Valencell Health Fingertip™ Blood Pressure Monitor - Video in English
Valencell Health Video (English) https://youtu.be/5qr2AihQzck
23 Jan 2024
Valencell Unveils Valencell Health™ and the Fingertip™ Blood Pressure Monitor The Calibration-Free, Cuffless BPM Solution Targeting Over-the-Counter Use
Raleigh, North Carolina, USA & Arab Health, Dubai, UAE – January 2024 – Valencell, the company whose optical heart rate technology enabled the ability of wearables to accurately measure cardiovascular vitals during exercise, today announced plans to launch its own branded product line in the digital health sector under the name Valencell Health™ as it concentrates efforts to bring solutions to market to manage chronic diseases. The company’s first product candidate is the Fingertip™ Blood Pressure Monitor, focused on helping people spot-check measure BP and manage hypertension by combining an intuitive app with an innovative over-the-counter device to accurately measure blood pressure (BP) from the finger without a cuff or calibration.
Founded in 2006 with a vision to help people live longer, healthier lives, Valencell’s proven inventions, sensor technology, and algorithms can be found in tens of millions of wearable and hearable devices, in over 90 products, for companies such as Samsung, Suunto, Bose, Jabra, Huawei, and Sony. While Valencell was established as a technology provider in consumer sports and fitness wearables, its foundational technology has the potential to realize the dream of seamless chronic disease management through easy-to-use, high-compliance digital health solutions.
The launch of the Valencell Health Fingertip™ BPM device will further the company’s vision of bringing life-changing digital health solutions to consumers and is a strategic evolution for the company. Having developed solutions for some of the world’s leading wearable companies, Valencell is now driven to bring its own portfolio of digital therapeutics to market. Powered by its patented biometric sensor technology, over 150 patents, proprietary AI models, and analytics that have been clinically validated using thousands of datasets over many years, it begins with a focus on hypertension.
“Having spent seventeen years perfecting the ability to capture health data from multiple areas of the body and developing solutions for third parties, I am excited for Valencell to embark on a new chapter,” said Dr. Steven LeBoeuf, President, and Co-Founder of Valencell. “Beginning with the number one silent killer, hypertension, Valencell is developing digital health solutions that take out all the friction and barriers to capturing and acting on health data to help make it easier for people to take control over their health, live longer, healthier, and more productive lives.”
Meeting Demands Under Pressure
Valencell’s first chronic disease management solution aims to help those with hypertension better and more regularly measure blood pressure and adhere to treatment.
The device has been engineered to measure blood pressure in less than a minute using a small probe on the middle finger without the need for bulky or painful cuffs. Photoplethysmography (PPG) sensors within the device use reflected light to measure blood flow patterns. Proprietary AI algorithms, developed from more than 20,000 PPG datasets comprising more than 10,000 patients, are used to process this information with physical characteristics (age, weight, height and biological sex) to calculate a blood pressure measurement. Diastolic and systolic results are then displayed on the device’s built-in screen and transmitted via Bluetooth® to the app.
Unlike other technologies, the Fingertip™ Blood Pressure Monitor does not need to be calibrated to a BP cuff, a capability that has so far eluded the industry. Valencell’s solution is expected to be the world’s first over-the-counter, cuffless and truly calibration-free blood pressure monitor in a fingertip form factor, with the company targeting FDA Clearance in late 2024. Altogether, this has the potential to enable the world’s first OTC cuffless BP monitoring solution.
Although the device has been designed as a stand-alone blood pressure monitor, it has the potential to become a powerful tool to help manage hypertension when paired with its connected app. The easy-to-use mobile app has been designed to track and store readings and allow users to set reminders to take their blood pressure. Users will also have the ability to view trends over time, track the frequency of their measurements, and download and share data.
The Heart of The Problem
Globally, more than a billion people suffer from hypertension, and it accounts for approx. ten million deaths per year, yet often hypertension has little or no symptoms and is difficult to manage on an ongoing basis. Critical to managing hypertension and saving lives are regular and reliable BP measurements. In a study of consumers that monitored BP at home, 80% of respondents stated they would increase the frequency of measurements using a fingertip device .
“The ability to accurately measure blood pressure is essential in ensuring those with cardiovascular conditions adhere to treatments and lifestyle changes their physicians recommend. Anything that makes this easier for people to do can save lives, dollars, and time,” commented Dr. Bill Kraus, Professor of Medicine, Duke University of Medicine. “The rise of digital health solutions and coaching apps are a welcomed trend but are only as good as the data that goes into them. Using the Valencell device that is as simple and painless as pulse oximeters could improve treatment adherence and outcomes and would be a welcomed solution to a largely unmet clinical need.”
Future of Digital Therapeutics
With new algorithms, form factors, and clinical data, blood flow patterns have the potential to measure health statistics ranging from respiratory rates, oxygen levels, cardiac efficiency, and blood pressure. The ability to place these capabilities into ubiquitous, everyday objects that are so intuitive they make it easy for consumers to manage their own health brings the promise of genuinely personal, preventative medicine close to reality.
To learn more about Valencell visit www.valencell.com. To experience it for yourself during Arab Health, please visit Valencell at booth H1.C51D in the US Pavillion, or contact Mark Felice at felice@velancell.com to schedule a demo..
###
About Valencell
Founded in 2006, and headquartered in Raleigh, North Carolina, USA, Valencell develops and delivers innovative, medical-grade digital health solutions focused on chronic disease management. Our intuitive, accurate solutions make it easier for consumers to manage their own health. Valencell’s patented biometric sensor technology, proprietary algorithms, and analytics, used by third-party wearable solutions on the market today, have been clinically validated in thousands of diverse participants over many years.
1 Valencell proprietary target audience study completed in October 2022 among 300 consumers ages 21-75 who monitored their own BP at home or at work.
Founded in 2006 with a vision to help people live longer, healthier lives, Valencell’s proven inventions, sensor technology, and algorithms can be found in tens of millions of wearable and hearable devices, in over 90 products, for companies such as Samsung, Suunto, Bose, Jabra, Huawei, and Sony. While Valencell was established as a technology provider in consumer sports and fitness wearables, its foundational technology has the potential to realize the dream of seamless chronic disease management through easy-to-use, high-compliance digital health solutions.
The launch of the Valencell Health Fingertip™ BPM device will further the company’s vision of bringing life-changing digital health solutions to consumers and is a strategic evolution for the company. Having developed solutions for some of the world’s leading wearable companies, Valencell is now driven to bring its own portfolio of digital therapeutics to market. Powered by its patented biometric sensor technology, over 150 patents, proprietary AI models, and analytics that have been clinically validated using thousands of datasets over many years, it begins with a focus on hypertension.
“Having spent seventeen years perfecting the ability to capture health data from multiple areas of the body and developing solutions for third parties, I am excited for Valencell to embark on a new chapter,” said Dr. Steven LeBoeuf, President, and Co-Founder of Valencell. “Beginning with the number one silent killer, hypertension, Valencell is developing digital health solutions that take out all the friction and barriers to capturing and acting on health data to help make it easier for people to take control over their health, live longer, healthier, and more productive lives.”
Meeting Demands Under Pressure
Valencell’s first chronic disease management solution aims to help those with hypertension better and more regularly measure blood pressure and adhere to treatment.
The device has been engineered to measure blood pressure in less than a minute using a small probe on the middle finger without the need for bulky or painful cuffs. Photoplethysmography (PPG) sensors within the device use reflected light to measure blood flow patterns. Proprietary AI algorithms, developed from more than 20,000 PPG datasets comprising more than 10,000 patients, are used to process this information with physical characteristics (age, weight, height and biological sex) to calculate a blood pressure measurement. Diastolic and systolic results are then displayed on the device’s built-in screen and transmitted via Bluetooth® to the app.
Unlike other technologies, the Fingertip™ Blood Pressure Monitor does not need to be calibrated to a BP cuff, a capability that has so far eluded the industry. Valencell’s solution is expected to be the world’s first over-the-counter, cuffless and truly calibration-free blood pressure monitor in a fingertip form factor, with the company targeting FDA Clearance in late 2024. Altogether, this has the potential to enable the world’s first OTC cuffless BP monitoring solution.
Although the device has been designed as a stand-alone blood pressure monitor, it has the potential to become a powerful tool to help manage hypertension when paired with its connected app. The easy-to-use mobile app has been designed to track and store readings and allow users to set reminders to take their blood pressure. Users will also have the ability to view trends over time, track the frequency of their measurements, and download and share data.
The Heart of The Problem
Globally, more than a billion people suffer from hypertension, and it accounts for approx. ten million deaths per year, yet often hypertension has little or no symptoms and is difficult to manage on an ongoing basis. Critical to managing hypertension and saving lives are regular and reliable BP measurements. In a study of consumers that monitored BP at home, 80% of respondents stated they would increase the frequency of measurements using a fingertip device .
“The ability to accurately measure blood pressure is essential in ensuring those with cardiovascular conditions adhere to treatments and lifestyle changes their physicians recommend. Anything that makes this easier for people to do can save lives, dollars, and time,” commented Dr. Bill Kraus, Professor of Medicine, Duke University of Medicine. “The rise of digital health solutions and coaching apps are a welcomed trend but are only as good as the data that goes into them. Using the Valencell device that is as simple and painless as pulse oximeters could improve treatment adherence and outcomes and would be a welcomed solution to a largely unmet clinical need.”
Future of Digital Therapeutics
With new algorithms, form factors, and clinical data, blood flow patterns have the potential to measure health statistics ranging from respiratory rates, oxygen levels, cardiac efficiency, and blood pressure. The ability to place these capabilities into ubiquitous, everyday objects that are so intuitive they make it easy for consumers to manage their own health brings the promise of genuinely personal, preventative medicine close to reality.
To learn more about Valencell visit www.valencell.com. To experience it for yourself during Arab Health, please visit Valencell at booth H1.C51D in the US Pavillion, or contact Mark Felice at felice@velancell.com to schedule a demo..
###
About Valencell
Founded in 2006, and headquartered in Raleigh, North Carolina, USA, Valencell develops and delivers innovative, medical-grade digital health solutions focused on chronic disease management. Our intuitive, accurate solutions make it easier for consumers to manage their own health. Valencell’s patented biometric sensor technology, proprietary algorithms, and analytics, used by third-party wearable solutions on the market today, have been clinically validated in thousands of diverse participants over many years.
1 Valencell proprietary target audience study completed in October 2022 among 300 consumers ages 21-75 who monitored their own BP at home or at work.
05 Jan 2024
About
Founded in 2006 and headquartered in Raleigh, North Carolina, USA
Valencell, the company whose optical heart rate technology enabled the ability of wearables to accurately measure cardiovascular vitals during exercise, plans to launch its own branded product line in the digital health sector under the name Valencell Health™ as it concentrates efforts to bring solutions to market to manage chronic diseases. The company’s first product candidate is the Fingertip™ Blood Pressure Monitor*, focused on helping people spot-check measure BP and manage hypertension by combining an intuitive app with an innovative over-the-counter device to accurately measure blood pressure (BP) from the finger without a cuff or calibration.
Founded in 2006 with a vision to help people live longer, healthier lives, Valencell’s proven inventions, sensor technology, and algorithms can be found in tens of millions of wearable and hearable devices, in over 90 products, for companies such as Samsung, Suunto, Bose, Jabra, Huawei, and Sony. While Valencell was established as a technology provider in consumer sports and fitness wearables, its foundational technology has the potential to realize the dream of seamless chronic disease management through easy-to-use, high-compliance digital health solutions.
The launch of the Valencell Health Fingertip™ BPM device will further the company’s vision of bringing life-changing digital health solutions to consumers and is a strategic evolution for the company. Having developed solutions for some of the world’s leading wearable companies, Valencell is now driven to bring its own portfolio of digital therapeutics to market. Powered by its patented biometric sensor technology, over 150 patents, proprietary AI models, and analytics that have been clinically validated using thousands of datasets over many years, it begins with a focus on hypertension.
Meeting Demands Under Pressure
Valencell Health’s first chronic disease management solution aims to help those with hypertension better and more regularly measure blood pressure and adhere to treatment.
The device has been engineered to measure blood pressure in less than a minute using a small probe on the middle finger without the need for bulky or painful cuffs. Photoplethysmography (PPG) sensors within the device use reflected light to measure blood flow patterns. Proprietary AI algorithms, developed from more than 20,000 PPG datasets comprising more than 10,000 patients, are used to process this information with physical characteristics (age, weight, height and biological sex) to calculate a blood pressure measurement. Diastolic and systolic results are then displayed on the device’s built-in screen and transmitted via Bluetooth® to the app.
Unlike other technologies, the Fingertip™ Blood Pressure Monitor does not need to be calibrated to a BP cuff, a capability that has so far eluded the industry. Valencell’s solution is expected to be the world’s first over-the-counter, cuffless and truly calibration-free blood pressure monitor in a fingertip form factor, with the company targeting FDA Clearance in late 2024. Altogether, this has the potential to enable the world’s first OTC cuffless BP monitoring solution.
Although the device has been designed as a stand-alone blood pressure monitor, it has the potential to become a powerful tool to help manage hypertension when paired with its connected app. The easy-to-use mobile app has been designed to track and store readings and allow users to set reminders to take their blood pressure. Users will also have the ability to view trends over time, track the frequency of their measurements, and download and share data.
Valencell, the company whose optical heart rate technology enabled the ability of wearables to accurately measure cardiovascular vitals during exercise, plans to launch its own branded product line in the digital health sector under the name Valencell Health™ as it concentrates efforts to bring solutions to market to manage chronic diseases. The company’s first product candidate is the Fingertip™ Blood Pressure Monitor*, focused on helping people spot-check measure BP and manage hypertension by combining an intuitive app with an innovative over-the-counter device to accurately measure blood pressure (BP) from the finger without a cuff or calibration.
Founded in 2006 with a vision to help people live longer, healthier lives, Valencell’s proven inventions, sensor technology, and algorithms can be found in tens of millions of wearable and hearable devices, in over 90 products, for companies such as Samsung, Suunto, Bose, Jabra, Huawei, and Sony. While Valencell was established as a technology provider in consumer sports and fitness wearables, its foundational technology has the potential to realize the dream of seamless chronic disease management through easy-to-use, high-compliance digital health solutions.
The launch of the Valencell Health Fingertip™ BPM device will further the company’s vision of bringing life-changing digital health solutions to consumers and is a strategic evolution for the company. Having developed solutions for some of the world’s leading wearable companies, Valencell is now driven to bring its own portfolio of digital therapeutics to market. Powered by its patented biometric sensor technology, over 150 patents, proprietary AI models, and analytics that have been clinically validated using thousands of datasets over many years, it begins with a focus on hypertension.
Meeting Demands Under Pressure
Valencell Health’s first chronic disease management solution aims to help those with hypertension better and more regularly measure blood pressure and adhere to treatment.
The device has been engineered to measure blood pressure in less than a minute using a small probe on the middle finger without the need for bulky or painful cuffs. Photoplethysmography (PPG) sensors within the device use reflected light to measure blood flow patterns. Proprietary AI algorithms, developed from more than 20,000 PPG datasets comprising more than 10,000 patients, are used to process this information with physical characteristics (age, weight, height and biological sex) to calculate a blood pressure measurement. Diastolic and systolic results are then displayed on the device’s built-in screen and transmitted via Bluetooth® to the app.
Unlike other technologies, the Fingertip™ Blood Pressure Monitor does not need to be calibrated to a BP cuff, a capability that has so far eluded the industry. Valencell’s solution is expected to be the world’s first over-the-counter, cuffless and truly calibration-free blood pressure monitor in a fingertip form factor, with the company targeting FDA Clearance in late 2024. Altogether, this has the potential to enable the world’s first OTC cuffless BP monitoring solution.
Although the device has been designed as a stand-alone blood pressure monitor, it has the potential to become a powerful tool to help manage hypertension when paired with its connected app. The easy-to-use mobile app has been designed to track and store readings and allow users to set reminders to take their blood pressure. Users will also have the ability to view trends over time, track the frequency of their measurements, and download and share data.
Add Attachment

Share
Recent Chats
Share via email
Future: handle WhatsApp here
Future: handle LinkedIn here
Future: handle Twitter here
SUBMENU HERE
Share via Chat
Copy Link




