Analyze Content
_300.webp)
ATLAS LINK TECHNOLOGY
Manassas, Virginia (VA), United States
Healthcare
Products & Services
People
About
Products & Services
Pregnancy test kits
Atlas Link Technology
Pregnancy test available in traditional test format (Strip, cassette, or Midstream) & NEW Biodegradable midstream.
Multi Panel infectious diseases
Atlas Link Technology
Multi Panel cassette that can detect multiple infectious diseases.
Combination can be made upon customers request
Combination can be made upon customers request
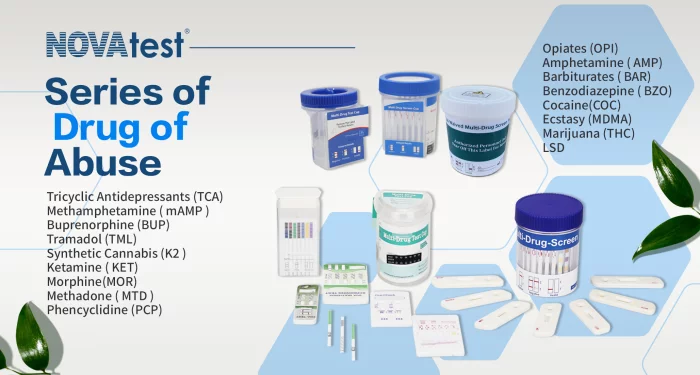
Drugs of abuse test
Atlas Link Technology
Drug of abuse test available in single or Multiple test in one.
Drug combination is made based on customers request
Drug combination is made based on customers request
About
Atlas Link (Beijing) Technology Co., Ltd. is incorporated in 2004, specializing in the design, research and development, production, and sales of in vitro diagnostic rapid test reagents and instruments.
Sales headquarter is located in Beijing, with independent sales branches in the United States, Singapore, and Hong Kong, Atlas Link Biotech has grown to become a large scientific and technological multinational corporation with over 600 employees globally. In recent years, the Company's business scope has expanded to include research and development, production, and application of high-tech materials, such as the new NOVAtest eco environmental protection portfolio and the third-party testing research center for medical devices, the Beta-Bear Laboratory. Atlas Link Biotech owns multiple internationally renowned trademarks, including NOVAtest and Sensotest, as well as dozens of patents and inventions.
Atlas Link Technology Co., Ltd. is established in 2010 and is situated in Langfang City, Hebei Province. It serves as the largest production base for in vitro diagnostic rapid test reagents under Atlas Link Biotech. The base covers a total area of 23,000 square meters, with multiple built-in 100,000-level clean production workshops and 10,000-level biochemistry laboratories, totaling 1,200 square meters of clean area. Through the implementation of fully semi-automated and partially automated processes, the production base is capable of reaching a monthly production capacity of 15-40 million tests. The base has more than 100 permanent employees, with over half possessing expertise in biomedical and business administration.
Offering more than 200 products, Atlas Link Biotech provides a range of in vitro diagnostic detection platforms, including colloidal gold immunochromatography, immunofluorescence, ELISA, dry chemistry, and more. The testing items cover fertility assessment, infectious disease, vector-borne disease, STD, marker, indicator and addictive substances, catering to Lab and POCT professional use as well as home self-testing. The majority of products have obtained CE certifications, FDA approvals, TGA certifications, and Chinese Class II medical device registration certificates.
Advanced Standardization Lab Unit is established as a research and development and testing laboratory solely funded by Atlas Link Biotech, aiming to facilitating the precise development of in vitro diagnostic reagents, reduce external dependence, and enhance the international competitiveness of products. The Lab Unit is an essential component of Atlas Link Biotech's R&D-Sales-Production strategic layout amd is the most revolutionary corporate structure innovation of Atlas Link Biotech in the last 20 years.
Equipped with a range of advanced equipment from Thermo Fisher Scientific, including biological safety cabinets, clean benches, microplate readers, cold storage solutions, microvolume UV-Vis spectrophotometers, and centrifuges, The Lab Unit is at the forefront of technological capability. The current team consists of over 10 technicians, with the R&D director boasting more than 10 years of research and development experience. Furthermore, the core members of the team have all graduated from the top universities in China and around the world. With more than 100 new products developed independently as of 2023, the Lab has proven to be a powerhouse of innovation.
In addition to its research and development capabilities, the Lab Unit has established a small internal GMP production workshop. This ensures that the design results can be swiftly transformed from the development process to finished products.
Sales headquarter is located in Beijing, with independent sales branches in the United States, Singapore, and Hong Kong, Atlas Link Biotech has grown to become a large scientific and technological multinational corporation with over 600 employees globally. In recent years, the Company's business scope has expanded to include research and development, production, and application of high-tech materials, such as the new NOVAtest eco environmental protection portfolio and the third-party testing research center for medical devices, the Beta-Bear Laboratory. Atlas Link Biotech owns multiple internationally renowned trademarks, including NOVAtest and Sensotest, as well as dozens of patents and inventions.
Atlas Link Technology Co., Ltd. is established in 2010 and is situated in Langfang City, Hebei Province. It serves as the largest production base for in vitro diagnostic rapid test reagents under Atlas Link Biotech. The base covers a total area of 23,000 square meters, with multiple built-in 100,000-level clean production workshops and 10,000-level biochemistry laboratories, totaling 1,200 square meters of clean area. Through the implementation of fully semi-automated and partially automated processes, the production base is capable of reaching a monthly production capacity of 15-40 million tests. The base has more than 100 permanent employees, with over half possessing expertise in biomedical and business administration.
Offering more than 200 products, Atlas Link Biotech provides a range of in vitro diagnostic detection platforms, including colloidal gold immunochromatography, immunofluorescence, ELISA, dry chemistry, and more. The testing items cover fertility assessment, infectious disease, vector-borne disease, STD, marker, indicator and addictive substances, catering to Lab and POCT professional use as well as home self-testing. The majority of products have obtained CE certifications, FDA approvals, TGA certifications, and Chinese Class II medical device registration certificates.
Advanced Standardization Lab Unit is established as a research and development and testing laboratory solely funded by Atlas Link Biotech, aiming to facilitating the precise development of in vitro diagnostic reagents, reduce external dependence, and enhance the international competitiveness of products. The Lab Unit is an essential component of Atlas Link Biotech's R&D-Sales-Production strategic layout amd is the most revolutionary corporate structure innovation of Atlas Link Biotech in the last 20 years.
Equipped with a range of advanced equipment from Thermo Fisher Scientific, including biological safety cabinets, clean benches, microplate readers, cold storage solutions, microvolume UV-Vis spectrophotometers, and centrifuges, The Lab Unit is at the forefront of technological capability. The current team consists of over 10 technicians, with the R&D director boasting more than 10 years of research and development experience. Furthermore, the core members of the team have all graduated from the top universities in China and around the world. With more than 100 new products developed independently as of 2023, the Lab has proven to be a powerhouse of innovation.
In addition to its research and development capabilities, the Lab Unit has established a small internal GMP production workshop. This ensures that the design results can be swiftly transformed from the development process to finished products.
Add Attachment

Share
Recent Chats
Share via email
Future: handle WhatsApp here
Future: handle LinkedIn here
Future: handle Twitter here
SUBMENU HERE
Share via Chat
Copy Link

