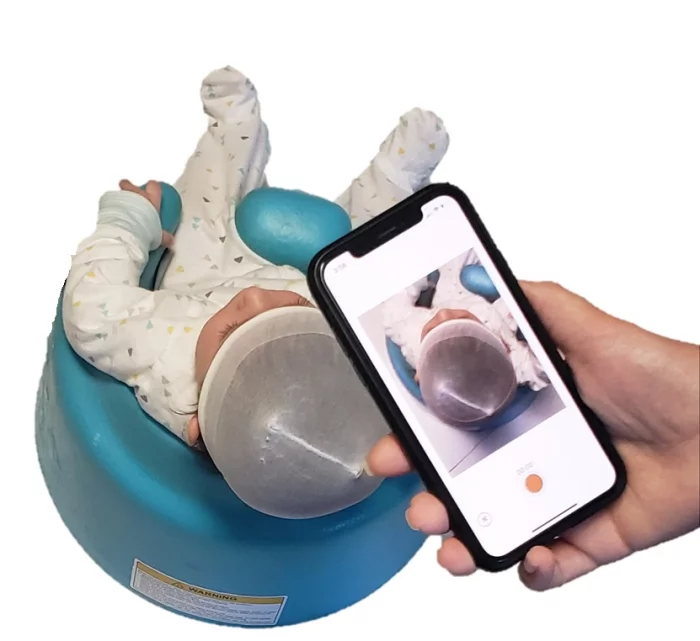
PediaMetrix gets FDA clearance for SoftSpot
The company received U.S. Food and Drug Administration clearance for its mobile app, which provides software tools for clinicians to measure and monitor the head of infants.
Details
More Products & Services
Description
The 510(k) clearance, which is designed for medical devices, covers all aspects of the platform: its fronted mobile apps and web platform, its backend AI and computer visions technology, and its AWS-based cloud platform.
“This is by far the biggest thing that can happen to a medical device/pharma company,” PediaMetrix CEO Dr. Fereshteh Aalamifar said. “FDA approval is a badge of honor showing you have satisfied a rigorous review process by the FDA, showing your device is safe and effective for the intended use.”
It means the company can move forward with bringing the technology to market in the U.S., and any other country that recognizes FDA clearance. The U.S. validation also eases the path to market in countries that have distinct regulatory clearance processes, including the European Union and Latin America. That’s important for PediaMetrix, as it is getting requests for the technology from English-speaking countries, Southeast Asia and Brazil.
Dr. Fereshteh Aalamifar. (Courtesy photo)
PediaMetrix was founded in 2018 by Aalamifar, who earned her Ph.D. at Baltimore’s Johns Hopkins University, and a group of scientists and entrepreneurs who are alumni of Johns Hopkins, Harvard University and Princeton University. It is designed to provide a tool for quantitative assessment of infants’ cranial conditions at the point of care. Currently, pediatricians use a tape measure for the measurement of head circumference only, and refer at-risk children to specialists, which can cause delays.
“Conditions with cranial deformity are on the rise and affect over 20% of newborns,” said Aalamifar, in a statement. “These conditions, including deformational plagiocephaly and brachycephaly and craniosynostosis, cause an estimated $1B cost to the US healthcare.”
Accessible for any smartphone, it allows pediatricians and parents to use the phone’s camera to take a short video, then uses image processing to make specific calculations about the head, and assists in the decision-making process for next steps. With data that demonstrated over 93% correlation with the standard cranial measurements used to determine whether patients have the conditions deformational plagiocephaly and brachycephaly, per the company, it is heading for a U.S. market launch in Q4 of 2021.
The approval will also lead to hiring. The company currently has 10 full-time and part-time team members in the U.S. and other countries. It is hiring in areas including data analysis, sales and marketing, and customer support. It will also seek quality assurance and reimbursement contractors in next few months, and is eyeing more R&D engineering hires in 2022.
Based out of the Rockville Innovation Center, PediaMetrix received investment from Maryland early-stage technology support agency TEDCO’s Builder Fund, as well as funding from the National Science Foundation through the Small Business Innovation Research program and Rockville-based BioHealth Innovation. It’s a graduate of accelerators including Reston, Virginia-based Startups Ignite and Annapolis, Maryland-based FounderTrac.
FDA clearance for software is evolving as tech develops, and PediaMetrix said its clearance is the first for a smartphone app category that’s specifically focused on cranial measurements. The approval comes around the same time as wider evolution of regulations at the Silver Spring-based government agency, as the FDA issued an action plan specifically geared toward AI and machine learning in software-as-medical device regulations in September.
“This is by far the biggest thing that can happen to a medical device/pharma company,” PediaMetrix CEO Dr. Fereshteh Aalamifar said. “FDA approval is a badge of honor showing you have satisfied a rigorous review process by the FDA, showing your device is safe and effective for the intended use.”
It means the company can move forward with bringing the technology to market in the U.S., and any other country that recognizes FDA clearance. The U.S. validation also eases the path to market in countries that have distinct regulatory clearance processes, including the European Union and Latin America. That’s important for PediaMetrix, as it is getting requests for the technology from English-speaking countries, Southeast Asia and Brazil.
Dr. Fereshteh Aalamifar. (Courtesy photo)
PediaMetrix was founded in 2018 by Aalamifar, who earned her Ph.D. at Baltimore’s Johns Hopkins University, and a group of scientists and entrepreneurs who are alumni of Johns Hopkins, Harvard University and Princeton University. It is designed to provide a tool for quantitative assessment of infants’ cranial conditions at the point of care. Currently, pediatricians use a tape measure for the measurement of head circumference only, and refer at-risk children to specialists, which can cause delays.
“Conditions with cranial deformity are on the rise and affect over 20% of newborns,” said Aalamifar, in a statement. “These conditions, including deformational plagiocephaly and brachycephaly and craniosynostosis, cause an estimated $1B cost to the US healthcare.”
Accessible for any smartphone, it allows pediatricians and parents to use the phone’s camera to take a short video, then uses image processing to make specific calculations about the head, and assists in the decision-making process for next steps. With data that demonstrated over 93% correlation with the standard cranial measurements used to determine whether patients have the conditions deformational plagiocephaly and brachycephaly, per the company, it is heading for a U.S. market launch in Q4 of 2021.
The approval will also lead to hiring. The company currently has 10 full-time and part-time team members in the U.S. and other countries. It is hiring in areas including data analysis, sales and marketing, and customer support. It will also seek quality assurance and reimbursement contractors in next few months, and is eyeing more R&D engineering hires in 2022.
Based out of the Rockville Innovation Center, PediaMetrix received investment from Maryland early-stage technology support agency TEDCO’s Builder Fund, as well as funding from the National Science Foundation through the Small Business Innovation Research program and Rockville-based BioHealth Innovation. It’s a graduate of accelerators including Reston, Virginia-based Startups Ignite and Annapolis, Maryland-based FounderTrac.
FDA clearance for software is evolving as tech develops, and PediaMetrix said its clearance is the first for a smartphone app category that’s specifically focused on cranial measurements. The approval comes around the same time as wider evolution of regulations at the Silver Spring-based government agency, as the FDA issued an action plan specifically geared toward AI and machine learning in software-as-medical device regulations in September.

Share
Recent Chats
Share via email
Future: handle WhatsApp here
Future: handle LinkedIn here
Future: handle Twitter here
SUBMENU HERE
Share via Chat
Copy Link