
SYLKE INC.
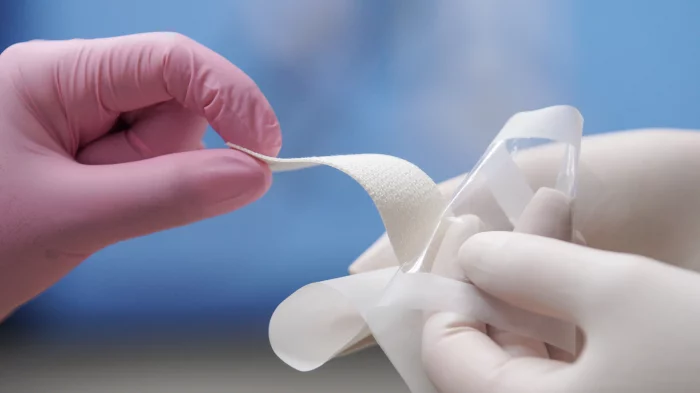
SYLKE® Skin Closure is indicated for use as a topical wound closure device and the treatment and coverage of thoroughly cleansed simple lacerations, cuts, puncture wounds, and post-surgical wounds: https://www.youtube.com/watch?v=M_W8vBEVuMU.
La Jolla, California (CA), United States
Healthcare
Products & Services
People
About
Products & Services
About
SYLKE® was developed by M. Mark Mofid M.D., FACS, a practicing reconstructive and plastic surgeon with more than 20 years of experience. Dr. Mofid sought to create a product that better addressed the needs of surgical incision care. During the early months of the COVID pandemic, with a lighter surgical schedule, he focused on designing a wound closure device using silk fibroin, a material known for its strength and biocompatibility. "I envisioned a comfortable and effective skin closure device that meets the needs of both patients and practitioners."
Add Attachment

Share
Recent Chats
Share via email
Future: handle WhatsApp here
Future: handle LinkedIn here
Future: handle Twitter here
SUBMENU HERE
Share via Chat
Copy Link

